Quantifying Lithium Brines with Lithium-7 Benchtop NMR Spectroscopy
The use of lithium around the world has increased drastically in recent years due to the superior performance of lithium-ion batteries. These batteries are heavily used in everyday life as they are present in our phones, computers, watches, vehicles, and many more devices. However, before these batteries can be produced, we need to procure lithium from somewhere. That “somewhere” is either spodumene or lithium-containing brines, with the latter being responsible for 59% of the market share of lithium production.2
The process of isolating lithium from brines is quite unique. The brines are concentrated in large pools using solar evaporation, with different minerals and compounds precipitating over time. The overall concentration process takes approximately 12-18 months before the brines are sufficiently concentrated to move to the next stage in processing. It is important to note that the lithium concentrations of these brines are monitored during the entire process.
To quantify the lithium content in these brines, techniques such as inductively coupled plasma – optical emission spectroscopy (ICP-OES) and atomic absorption (AA) spectroscopy are typically used. However, these methods require significant sample manipulation and are prone to the interference of other ions and compounds in the mixture. This interference results in reduced accuracy when quantifying brines. In a previous blog post, we discussed the potential for lithium quantification using benchtop NMR, and in this blog post, we will show that benchtop NMR can be used to quantify real brine samples.
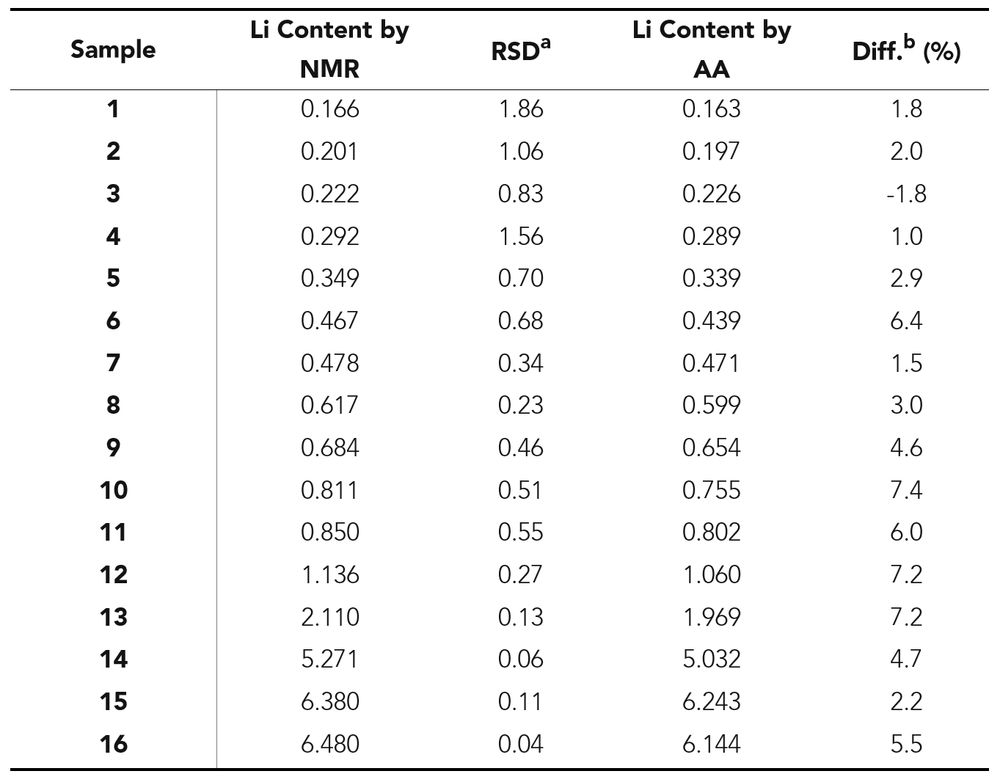
In collaboration with Sociedad Química y Minera (SQM) in Chile, 16 brine samples were simultaneously analyzed by quantitative NMR (qNMR) using a benchtop NMR spectrometer at Nanalysis’ headquarters in Calgary, Alberta, Canada, and using AA spectroscopy at SQM. The results were then compared in the table below.
Table 1. Lithium content determined using 7Li qNMR and AA spectroscopy in brine samples. All concentrations are expressed as w/w% lithium.
a Relative standard deviation of NMR measurements. b((Li content by NMR – Li content by AA spectroscopy)/(Li content by AA spectroscopy)) x 100.
NMR spectroscopy provides sufficient sensitivity to analyze these real brine samples, and, unlike ICP-OES and AA spectroscopy, this approach does not require significant sample preparation or dilution for lithium determination. This makes benchtop NMR an attractive new method for quantification of lithium in the mining industry. If you would like to know more about lithium quantification using benchtop NMR spectroscopy or are interested in how this technology could help you, please do not hesitate to contact us or check our recent publication.
References
[1] J. F. Araneda, P. Hui, G.M. Leskowitz, S.D. Riegel, R. Mercado, and C. Green, Analyst., 2021, 146, 882 - 888
[2] P. Meshram, B. D. Pandey and T. R. Mankhand, Hydrometallurgy, 2014, 150, 192 —208