The Wonders of Fluorine-19 NMR Spectroscopy for Pesticide Analysis
From industry to academia, Nuclear Magnetic Resonance (NMR) spectroscopy is a pillar in cementing valuable applications for qualitative and quantitative analyses of chemical compounds. With a broad range of experiments to choose from, one can analyze chemicals in a myriad of ways which provides different information on the sample of interest.
With a natural isotopic abundance of 100%, fluorine-19 can be a valuable nuclide to analyze fluorinated compounds. Besides the pharmaceutical industry, the agrochemical industry also benefits from using 19F NMR spectroscopy to analyze its respective compounds. For example, the pesticide industry utilizes 19F NMR to analyze the concentration of active ingredients as a quality check in the products they produce. Furthermore, fluorine has an increasing importance in agricultural chemistry as fluorinated groups (e.g., CF3, CHF2, F) add stability and lipophilicity to pesticides.1
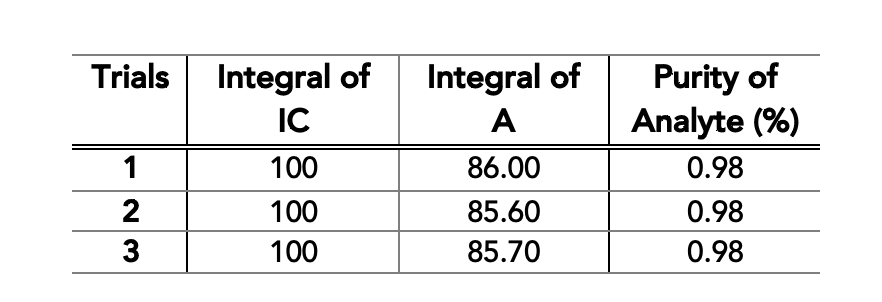
Fipronil is a fluorinated compound developed as pesticide used in both agriculture and domestic health.2 The pesticide in the spectra below contains fipronil as an active ingredient. Since pesticides are mixtures of organic chemicals, the 1H NMR spectrum (Figure 1) is quite complex relative to the 19F NMR spectrum. A quantitative 19F NMR spectrum (Figure 2) can be acquired provided that the internal calibrant has a sufficient purity and the peaks do not interfere with the other signals of interest in the sample.3 For this pesticide, fipronil produces two signals from the two CF3 groups and the internal standard, 4,4’-difluorobenzophenone, due to its symmetry produces one signal. Due to fluorine having a larger spectral window relative to other nuclei like proton, the signal overlap is minimized. With this mode of analysis, we can quantify the amount of the active ingredient in the sample in a very short time (Table 1).
Figure 1. 1H NMR spectrum of sample pesticide in chloroform-d.
Figure 2. 19F (96 MHz) NMR spectrum of sample pesticide with the addition of the internal calibrant, 4,4’-difluorobenzophenone, in chloroform-d, used for the weight percentage of fipronil in the pesticide.
Table 1. Determined weight percentages of fipronil in the sample.
With the advent of benchtop NMR instruments, regulators and producers in the agrochemical industry can benefit from the existing methods in 19F NMR spectroscopy to analyze pesticides in order to develop more effective formulae. To see how other heteronuclides like phosphorus can be useful in analyzing pesticides, click here. If you have any questions about the incorporation of benchtop NMR into your workflow, please don’t hesitate to contact us!
References
[1] Mabury S. A.; Crosby, D. G., Journal of Agricultural and Food Chemistry 1995, 43(7), 1845-1848.
[2] Magalhães, J. Z., et al. "Fipronil: uses, pharmacological and toxicological features. Revinter 2018 , 11.1, 67-83.
[3] Pauli, G. F.; Chen, S. N.; Simmler, C.; Lankin, D. C.; Gödecke, T.; Jaki, B. U.; Friesen, J. B.; McAlpine, J. B.; Napolitano, J. G. J. Med. Chem. 2014, 57, 9220–9231.